Symptoms of cystic fibrosis (CF) can include very salty-tasting skin, persistent coughing, frequent lung infection, wheezing, shortness of breath, poor growth, gradual weight gain (despite a healthy diet), and frequently greasy, bulky, or difficult stools. There is an estimated 30,000 children and adults diagnosed with CF in the US, 70,000 world wide. Testing for cystic fibrosis can be handled in several ways, including sweat tests, newborn screening, and carrier testing. Positive results on these tests could lead to a diagnosis for CF.
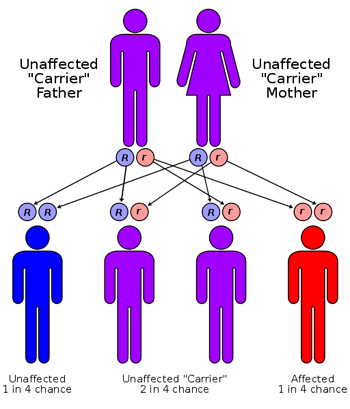
Cystic fibrosis is passed down genetically when a child inherits one copy of the defective gene from each parent. CF is a recessive gene, which means that a person must have both copies of the mutated gene in order to be affected by cystic fibrosis.
In a non-defective gene, the protein acts as a gateway, allowing ions to bring water to the surface of the airway. This keeps the mucus moist. In CF patients, the vital chloride channel is blocked, preventing the movement of chloride ions into the mucus.
This then halts the movement of water, causing the mucus to dry out. The mucus builds and begins to clog the lungs and obstruct the pancreas.
While there is no known cure for cystic fibrosis, there are several options for treatments that will reduce the symptoms and their effects. There are several medication options for CF treatment, including antibiotics, mucus-thinning drugs, bronchodilators, and oral pancreatic enzymes. Other treatment options include chest physical therapy, pulmonary rehabilitation, and surgical or other procedures.
When a child or loved one is diagnosed with CF, it is very important to care for them and offer full support. There are several ways to bring aid to a CF patient, including encouraging better nutrition, heightened fluid intake, updated immunizations, exercise, and hand washing. It is also vital to illuminate smoke from the patient's surroundings, as it will only irritate the respiratory tract and increase the issue.